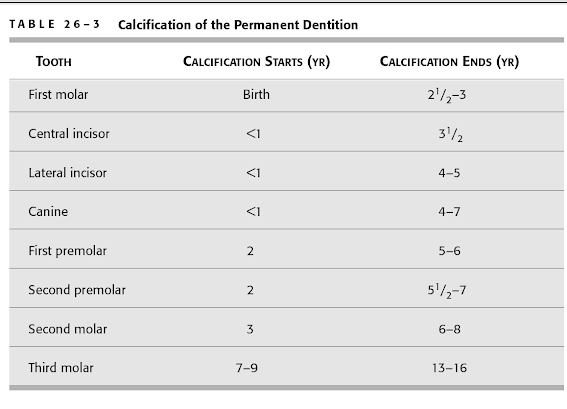
# Which teeth are least affected with the nursing bottle syndrome?
A. Maxillary molars
B. Maxillary and mandibular canines
C. Mandibular incisors
D. Maxillary incisors
The correct answer is C. Mandibular incisors.
EARLY CHILDHOOD CARIES
Early childhood caries was historically attributed to inappropriate and prolonged use of sweetened liquid in the bottle. Hence the older terms of “baby-bottle tooth decay” and “nursing caries. Early caries involvement of the maxillary anterior teeth, the maxillary and mandibular posterior teeth and mandibular canines is seen.
FEATURES:
• Mandibular incisors are unaffected due to the protection by the tongue.
• Seen as white or dark brown collar of caries around the neck o f the incisors, which develops into
faciolingual caries and may also fracture the tooth.
• The main etiology is that the child is put to bed with a nursing bottle containing milk or sugar containing beverages. The child falls asleep and the milk or sweetened liquid becomes pooled around the maxillary anterior teeth. This provides an excellent culture medium for acidogenic microorganisms.
Salivary flow is reduced during sleep and clearance of the liquid from the oral cavity is slowed.